Are you scouring the internet for 'kinetics of the acid catalyzed iodination of propanone essay'? Here you will find all the details.
Table of contents
- Kinetics of the acid catalyzed iodination of propanone essay in 2021
- Ch3coch3 + i2
- Why is iodine not in the rate equation
- Iodination of propanone mechanism
- Iodination of propanone lab report
- Iodination of propanone chemistry ia
- Iodination of propanone rate equation
- Iodine propanone reaction titrimetric method
Kinetics of the acid catalyzed iodination of propanone essay in 2021
 This picture representes kinetics of the acid catalyzed iodination of propanone essay.
This picture representes kinetics of the acid catalyzed iodination of propanone essay.
Ch3coch3 + i2
 This picture illustrates Ch3coch3 + i2.
This picture illustrates Ch3coch3 + i2.
Why is iodine not in the rate equation
 This picture shows Why is iodine not in the rate equation.
This picture shows Why is iodine not in the rate equation.
Iodination of propanone mechanism
 This picture shows Iodination of propanone mechanism.
This picture shows Iodination of propanone mechanism.
Iodination of propanone lab report
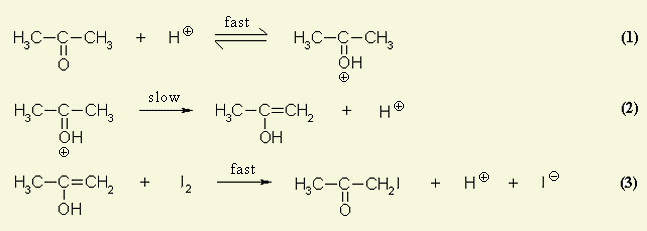 This image demonstrates Iodination of propanone lab report.
This image demonstrates Iodination of propanone lab report.
Iodination of propanone chemistry ia
 This picture shows Iodination of propanone chemistry ia.
This picture shows Iodination of propanone chemistry ia.
Iodination of propanone rate equation
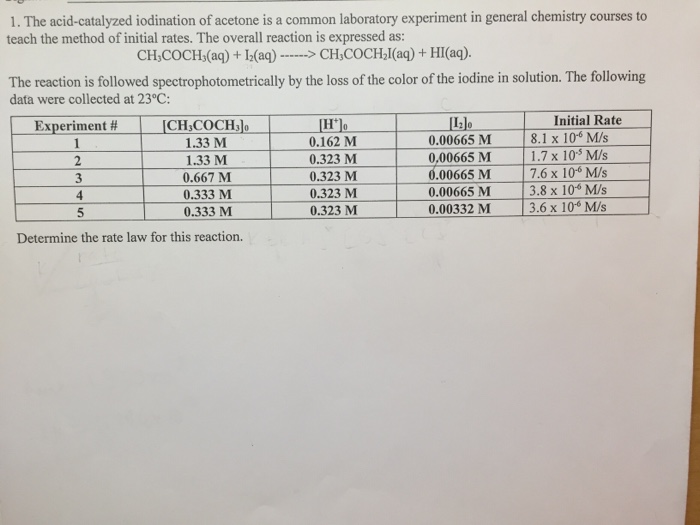 This picture illustrates Iodination of propanone rate equation.
This picture illustrates Iodination of propanone rate equation.
Iodine propanone reaction titrimetric method
 This picture representes Iodine propanone reaction titrimetric method.
This picture representes Iodine propanone reaction titrimetric method.
How to calculate the iodination of propanone in a colorimeter?
Place a cuvette full of distilled water inside the colorimeter and press the zero the button. Select a suitable filter (gives your greatest concentration an absorption of close to one). Mix sample A, sample B and 2 ml of 0.02 mol dm -3 HCL in a cuvette (add sample A last).
How is the positive charge transferred from propanone to oxygen?
The mechanism for propanone, with only one slow step suggested is ... The ketone is reversibly protonated on the oxygen (+) by the acid in an acid–base reaction (proton transfer). The electrons 'between' the C–O partly shift to form a carbocation i.e. the positive charge is transferred from the oxygen to the carbon.
Which is product of acid catalyzed iodination of propanone?
Acid-catalyzed iodination of propanone is a spontaneous halogenation reaction between propanone and iodine in an acidic environment, forming colorless iodopropanone and hydrogen iodide as products.
How is the iodine reaction described on the periodic table?
This reaction is described as autocatalytic as it is produces the very species that increases the rate of reaction (hydrogen ions 7 ). Iodine is chemical that takes place in the reaction. Iodine is a halogen (group 7 on the periodic table) with a simple molecular covalent structure.
Last Update: Oct 2021