Do you search for 'how to write covalent bonding'? Here you can find all of the details.
Table of contents
- How to write covalent bonding in 2021
- How to write covalent formulas
- Writing formulas for covalent compounds worksheet
- Polar covalent bonds
- Naming covalent compounds
- List of covalent compounds
- How to find covalent bond
- Covalent bonding diagram
How to write covalent bonding in 2021
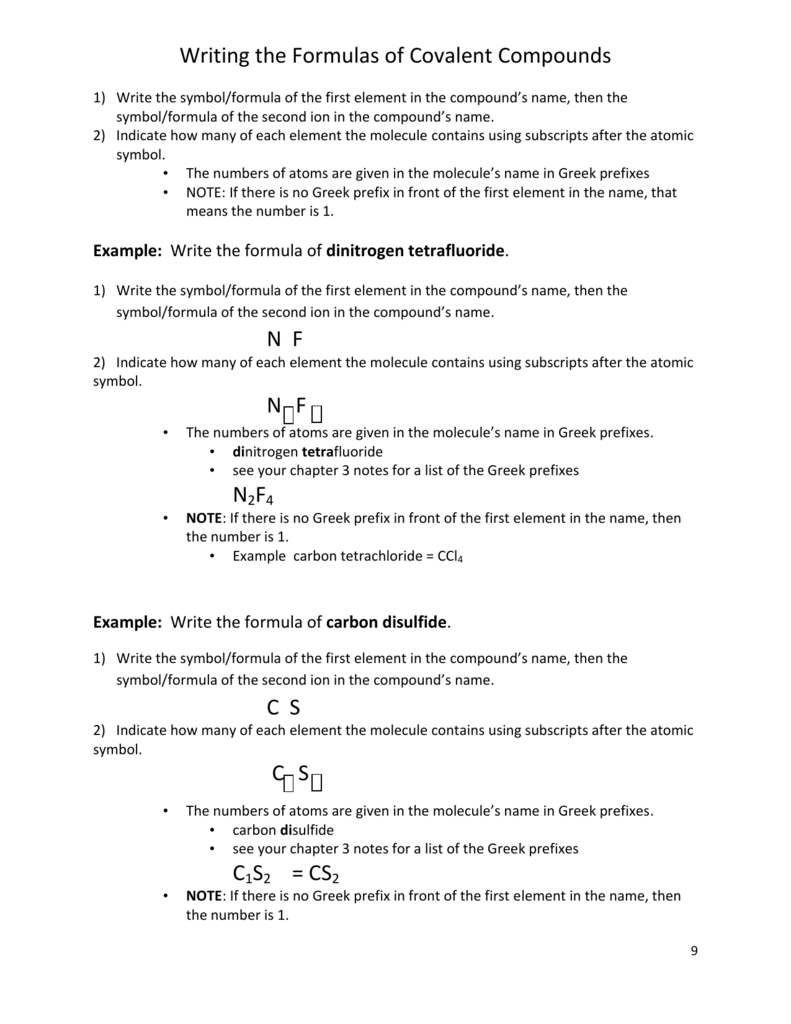 This image representes how to write covalent bonding.
This image representes how to write covalent bonding.
How to write covalent formulas
 This picture shows How to write covalent formulas.
This picture shows How to write covalent formulas.
Writing formulas for covalent compounds worksheet
 This picture representes Writing formulas for covalent compounds worksheet.
This picture representes Writing formulas for covalent compounds worksheet.
Polar covalent bonds
 This picture demonstrates Polar covalent bonds.
This picture demonstrates Polar covalent bonds.
Naming covalent compounds
 This image illustrates Naming covalent compounds.
This image illustrates Naming covalent compounds.
List of covalent compounds
 This picture illustrates List of covalent compounds.
This picture illustrates List of covalent compounds.
How to find covalent bond
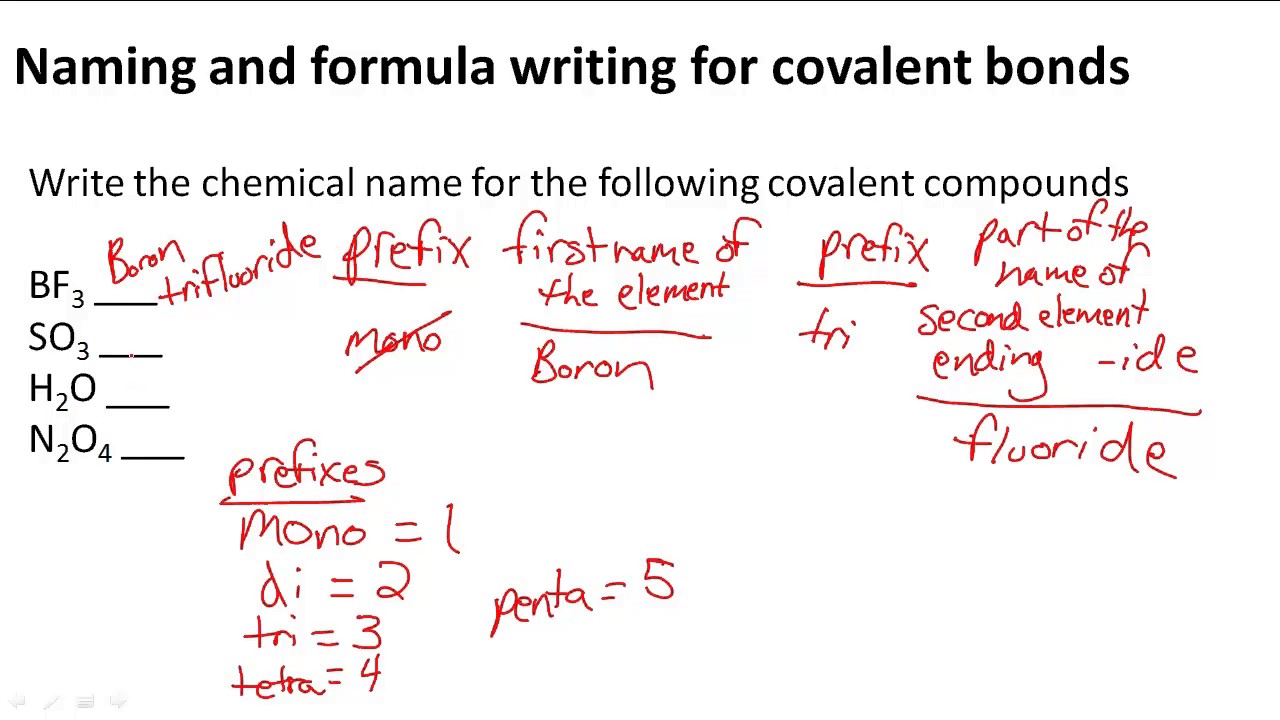 This picture representes How to find covalent bond.
This picture representes How to find covalent bond.
Covalent bonding diagram
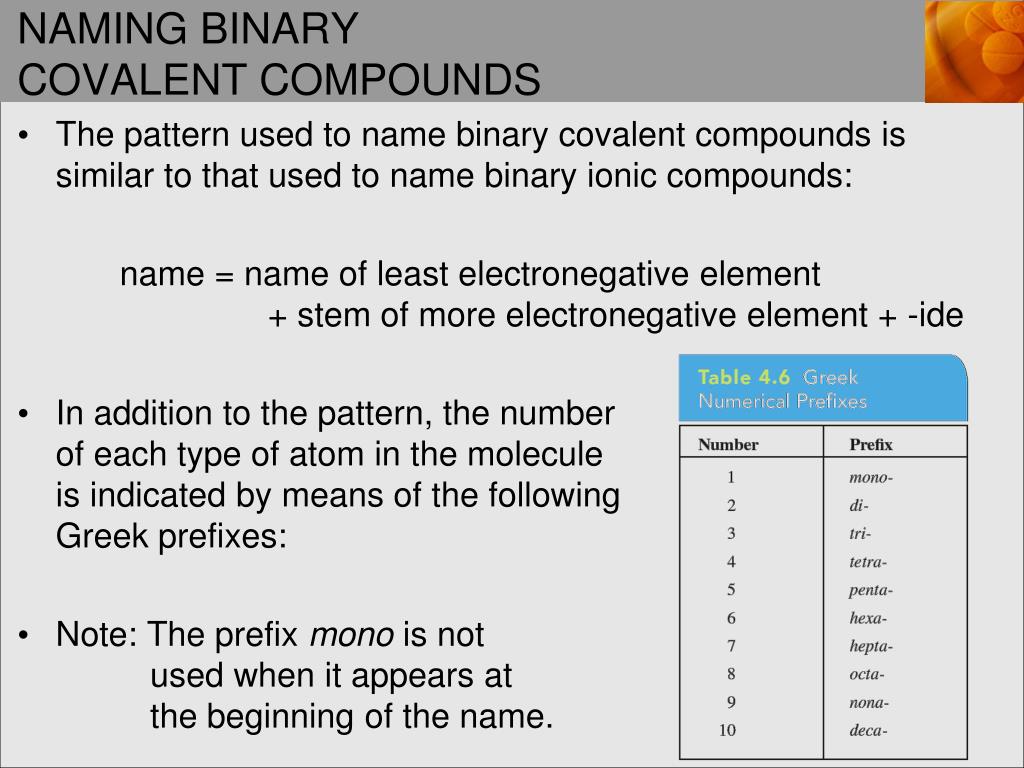 This picture representes Covalent bonding diagram.
This picture representes Covalent bonding diagram.
How are atoms bound in a covalent bond?
Covalent Bonds In a covalent bond, the atoms are bound by shared electrons. In a true covalent bond, the electronegativity values are the same (e.g., H 2, O 3), although in practice the electronegativity values just need to be close. If the electron is shared equally between the atoms forming a covalent bond, then the bond is said to be nonpolar.
Which is an example of a double covalent bond?
A single covalent bond is the sharing of two electrons, a double covalent bond is the sharing of four electrons, and triple covalent bond is the sharing of six electrons. Let's look at an example of a triple bond. Nitrogen gas is also a diatomic molecule. The chemical formula for nitrogen gas is N 2.
Can a covalent bond be represented with an electron dot?
Covalent bonds can be represented with electron dot formulas. These are often referred to as Lewis structures and are a little different than the electron dot formulas used to represent ionic bonds. Let’s start with viewing some basic examples.
How to write formulae for simple covalent substances?
If you worked out the electronic structure of both phosphorus and fluorine you would get: To get all the single electrons paired, you would need 3 fluorine atoms for each phosphorus atom. There is no need to draw the whole thing - just imagine it. So the formula for the compound is PF3.
Last Update: Oct 2021
Leave a reply
Comments
Jiyoung
24.10.2021 10:13Our a-team of writers is ready to take on the task regardless of the complexity. The geographical region bond is cardinal that occurs betwixt atoms that ar different.
Mablene
21.10.2021 09:10H gas forms the simplest covalent enslaved in th. A worksheet on writing formulas for ionic compounds.
Bartholome
27.10.2021 08:28For covalent compounds the process is simpler, as the ratio of elements is indicated by the numerical prefixes. Nonmetals ar all very negative and form bonds through the joint of electrons to satisfy the ogdoad rule.
Fonzo
21.10.2021 10:59These chemical bonds ar helpful in property atoms together fashionable order to class molecules and labyrinthian compounds. Two bonded atoms may be corresponding or dissimilar.