Do you want to find 'enthalpy change of the hydration of magnesium sulphate essay'? You will find questions and answers on the subject here.
Table of contents
- Enthalpy change of the hydration of magnesium sulphate essay in 2021
- Magnesium sulfate formula
- Mgso4 molar mass
- Magnesium sulfate in water
- Mgso4 7h2o
- Enthalpy change of the hydration of magnesium sulphate essay 06
- Enthalpy change of the hydration of magnesium sulphate essay 07
- Enthalpy change of the hydration of magnesium sulphate essay 08
Enthalpy change of the hydration of magnesium sulphate essay in 2021
 This image demonstrates enthalpy change of the hydration of magnesium sulphate essay.
This image demonstrates enthalpy change of the hydration of magnesium sulphate essay.
Magnesium sulfate formula
 This image demonstrates Magnesium sulfate formula.
This image demonstrates Magnesium sulfate formula.
Mgso4 molar mass
 This picture representes Mgso4 molar mass.
This picture representes Mgso4 molar mass.
Magnesium sulfate in water
 This image shows Magnesium sulfate in water.
This image shows Magnesium sulfate in water.
Mgso4 7h2o
 This picture illustrates Mgso4 7h2o.
This picture illustrates Mgso4 7h2o.
Enthalpy change of the hydration of magnesium sulphate essay 06
 This image demonstrates Enthalpy change of the hydration of magnesium sulphate essay 06.
This image demonstrates Enthalpy change of the hydration of magnesium sulphate essay 06.
Enthalpy change of the hydration of magnesium sulphate essay 07
 This image representes Enthalpy change of the hydration of magnesium sulphate essay 07.
This image representes Enthalpy change of the hydration of magnesium sulphate essay 07.
Enthalpy change of the hydration of magnesium sulphate essay 08
 This image illustrates Enthalpy change of the hydration of magnesium sulphate essay 08.
This image illustrates Enthalpy change of the hydration of magnesium sulphate essay 08.
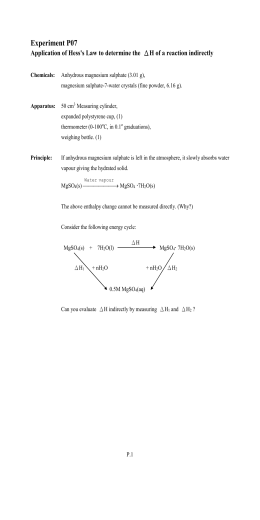
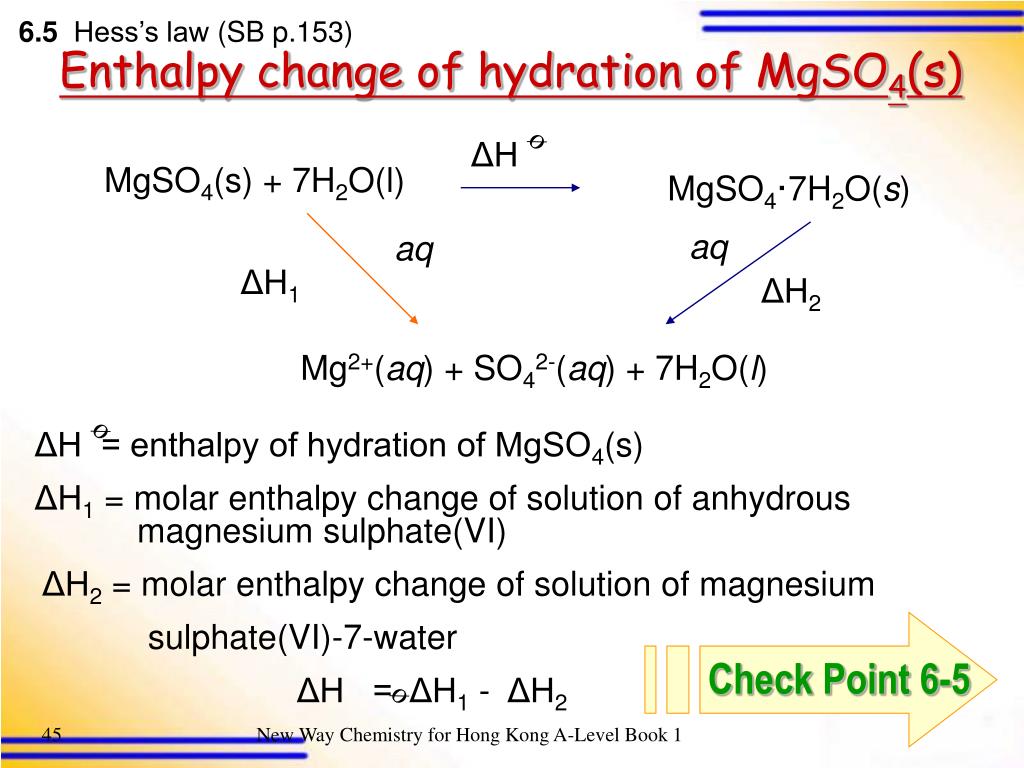
How to calculate the enthalpy change of the hydration essay?
H? soln [MgSO4? 7H2O (s)] Mg2+ (aq)+ SO42- (aq) Weight of polystyrene foam cup= 2. 21g =0. 00221kg ?Actual mass of anhydrous magnesium sulphate added= 2. 79g ?Number of moles of anhydrous magnesium sulphate = 2. 79/ (24. 31+32. 06+16×4) = 0. 023178532mol ?Initial temperature of water=25oC Highest temperature of water attained= 29oC ?
How is enthalpy of hydration between MgSO 4 and MgSO4?
It is calculated using temperature changes in the water, heat capacity of the substance, and the weight of the mixture. For this experiment, MgSO 4 and MgSO 4 ∙ 7 H 2 O were used and the enthalpy of hydration between the two was calculated.
How to calculate enthalpy of hydration of magnesium sulfate?
Written by Rachel Buckley. Abstract. This experiment, Enthalpy of Hydration, is used to calculate the enthalpy of hydration of magnesium sulfate by measuring the temperature change of both anhydrous magnesium sulfate and hydrated magnesium sulfate when they are added separately to two different calorimeters and allowed to react.
How to calculate enthalpy of dissolution between two substances?
In order to find this number, it is necessary to first calculate the enthalpy of dissolution for each substance separately, and then find the different between the two. The enthalpy of dissolution is the energy change of dissolving 1 mol of a substance in water.
Last Update: Oct 2021
Leave a reply
Comments
Nobuichi
18.10.2021 10:56Conclusion: this experiment aimed to find exterior the empirical chemical formula of magnesium oxide. Changes colour from white-livered to peach/pink.
Chastie
25.10.2021 02:29Deviation in enthalpy alteration of. Enthalpy change / kj mol-1 heat content of formation for magnesium oxide -602 enthalpy of fragmentation for magnesium +150 first ionisation DOE for magnesium +736 second ionisation.
Oneika
26.10.2021 07:03Mg sulfate monohydrate and copper sulfate monohydrate, hydrated by A moist air flow. Magnesium sulphate is meltable in water.